
Insights+: EMA Marketing Authorization of New Drugs in June 2023
Shots:
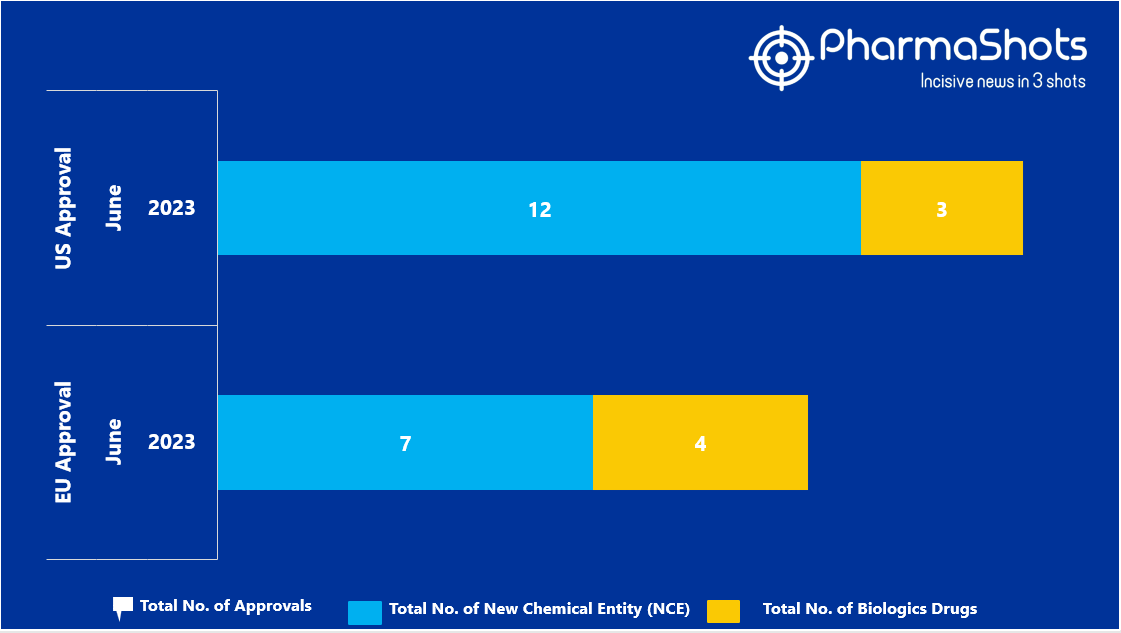
- The EMA approved 7 New Chemical Entity (NCE) and 4 Biologic Drugs in June 2023, leading to treatments for patients and advances in the healthcare industry
- In June 2023, the major highlights drugs were Jardiance approval for CKD patients, Briumvi (ublituximab-xiiy) for relapsing forms of multiple sclerosis
- PharmaShots has compiled a list of a total of 11 new drugs approved by the EMA in June 2023
Briumvi
Active ingredient: ublituximab-xiiy Approved: June 02, 2023
Company: TG Therapeutics Disease: Multiple Sclerosis
- The EC has approved Briumvi for adult patients with RMS with active disease defined by clinical or imaging features. The approval was based on the P-III trials (ULTIMATE I & II) evaluating Briumvi vs teriflunomide in 1094 patients across 10 countries
- The trials were led by Lawrence Steinman, MD, Zimmermann Professor of Neurology & Neurological Sciences, and Pediatrics at Stanford University. The results demonstrated superiority over teriflunomide in reducing the ARR, the no. of T1 Gd-enhancing lesions, and new or enlarging T2 lesions
- Briumvi (anti-CD20 mAb) was approved in the US & EU for RMS that can be administered in 1hr. infusion following the starting dose. The centralized marketing authorization is valid in all EU Member States, Iceland, Norway & Liechtenstein
Pedmarqsi
Active ingredient: sodium thiosulfate Approved: June 02, 2023
Company: Fennec Pharmaceuticals Disease: Cisplatin-Induced Ototoxicity
- The EC has granted the marketing authorization for Pedmarqsi to prevent ototoxicity (hearing loss) induced by cisplatin CT in patients aged 1mos. to <18yrs. with localized, non-metastatic solid tumors
- The decision was based on 2 P-III trials (SIOPEL 6) & (COG Protocol ACCL0431) evaluating Pedmarqsi + cisplatin-based regimen vs cisplatin-based regimens alone. The results showed that the hearing loss incidence rate was lower in the Pedmarqsi + cisplatin arm vs cisplatin alone (28.6% vs 56.4%) in (COG ACCLO431) & 35.1% vs 67.3% in (SIOPEL6) studies
- The marketing authorization is valid for all 27 EU member states, Iceland, Norway, and Liechtenstein. Pedmarqsi is currently marketed as Pedmark in the US
Bimzelx
Active ingredient: bimekizumab Approved: June 07, 2023
Company: UCB Disease: Psoriatic Arthritis and Axial Spondyloarthritis
- The approval was based on the P-III studies (BE OPTIMAL & BE COMPLETE) in 1252 patients with PsA evaluating bimekizumab (160mg, q4w) & (BE MOBILE 1 & 2) trial in 586 patients with active axSpA
- Both trials met its 1EPs & 2EPs i.e., patients with bimekizumab in PsA achieved ACR50 response in bDMARD-naive & TNFi-IR patients (44% vs 10%) & (43% vs 7%); MDA (45% vs 13%) & (44% vs 6%) @16wk.; patients with baseline psoriasis affecting ≥3 percent body surface area achieved complete skin clearance (PASI100) in (47% vs 2%) & (59% vs 5%), respectively. Clinical responses were sustained up to 52wk. in (BE OPTIMAL) trial
- In nr-axSpA & AS populations, ASAS40 response (47.7% vs 21.4%) & (44.8% vs 22.5%); low disease activity (46.2% vs 20.6%) & (44.9% vs 17.5%) @16wk., 6 out of 10 patients achieved ASDAS<2.1 @52wk., reduction of objective inflammatory signs in sacroiliac joints & spine @16 & 52wk.
Arexvy
Active ingredient: AS01E adjuvant Approved: June 07, 2023
Company: GSK Disease: Lower Respiratory Tract Disease
- The EC has authorized Arexvy, the first RSV vaccine for the prevention of LRTD caused by a respiratory syncytial virus (RSV) in adults aged ≥60yrs. The company plans to launch Arexvy before the 2023/24 RSV season
- The authorisation is based on the P-III trial (AReSVi-006) evaluating Arexvy in older adults aged ≥60yrs. The trial met its 1EPs & showed an overall vaccine efficacy of 82.6% against RSV-LRTD in adults aged ≥60yrs.; efficacy was 94.6% in older adults with one underlying medical condition of interest, was generally well tolerated
- The vaccine was also approved in the US while regulatory reviews are ongoing in Japan & multiple other countries
Trodelvy
Active ingredient: sacituzumab govitecan Approved: June 23, 2023
Company: GSK Disease: Metastatic Breast Cancer
- The EMA’s CHMP has adopted a positive opinion for Trodelvy as monotx. for adult patients with unresectable or metastatic HR+, HER2- breast cancer. The EC’s decision on an additional indication is expected in 2023
- The opinion was based on the P-III study (TROPiCS-02) evaluating Trodelvy vs CT in 543 patients which showed a clinical OS benefit of 3.2mos., m-OS (14.4 vs 11.2mos.), 34% reduction in risk of disease progression or death, m-PFS (5.5 vs 4.0mos.), patients were progression-free at 1yr. (21% vs. 7%)
- The therapy showed improved additional 2EPs measures, incl. ORR & TTD with no significant difference in TTD in pain scale. The safety profile was consistent with prior studies with no new safety signals, treatment discontinuation rate due to adverse reactions was 6% vs 4%
Qulipta
Active ingredient: Atogepant Approved: June 23, 2023
Company: AbbVie Disease: Migraine
- The EMA’s CHMP has adopted the positive opinion recommending the approval of atogepant for the prophylaxis of migraine in adults who have 4 or more migraine days per month
- The opinion was based on the P-III studies (PROGRESS) in 778 patients and (ADVANCE) in 910 patients evaluating atogepant vs PBO in chronic and episodic migraine. Both trials met their 1EPs & showed a reduction in mean monthly migraine days (MMDs) across the 12wk. treatment period, improvements in all 2EPs were seen & was well tolerated
- If atogepant is approved by EC, it will be the first oral calcitonin gene-related peptide (CGRP) receptor antagonist in the EU for the prophylaxis of migraine in adults
Amicus Therapeutics’ Pombiliti + Opfolda Receive EC’s Approval for the Treatment of Pompe Disease
Pombiliti and Opfolda
Active ingredient: cipaglucosidase alfa + miglustat Approved: June 25, 2023
Company: Amicus Therapeutics Disease: Pompe Disease
- The EC has approved Opfolda (65mg capsules) & Pombiliti for adults with LOPD. The company plans to launch Pombiliti + Opfolda in Germany & plans for reimbursement processes with healthcare authorities in other EU countries
- The approval was based on the P-III study (PROPEL) in ERT-naïve & ERT-experienced patients The results showed that Pombiliti + Opfolda exhibited clinically meaningful & positive changes in the key mobility & respiratory manifestations of the disease
- Pombiliti, a bis-M6P-enriched rhGAA enzyme that is designed for increased uptake into muscle cells. Opfolda is an enzyme stabilizer designed to stabilize the enzyme in the blood while Pombiliti + Opfolda received the BTD from the US FDA; MHRA regulatory approval is expected in Q3’23 & US FDA review is ongoing
Jardiance
Active ingredient: empagliflozin Approved: June 26, 2023
Company: Boehringer Ingelheim & Eli Lilly Disease: Chronic Kidney Disease
- The EC has approved Jardiance for adults with CKD. The approval was based on the P-III trial (EMPA-KIDNEY) evaluating the effect of Jardiance in 6609 adults from 8 countries
- The results showed a significant benefit of empagliflozin in reducing the relative risk of kidney disease progression or cardiovascular death by 28% with an absolute risk reduction of 3.8%, 14% relative risk reduction in hospitalization for any cause with absolute risk reduction (4.4%). The overall safety data was consistent with prior results confirming the well-established safety profile of empagliflozin
- Jardiance, the first SGLT2 inhibitor to show a significant reduction in all-cause hospitalizations. The product is expected to be available as quickly as possible
Soliris
Active ingredient: eculizumab Approved: June 27, 2023
Company: AstraZeneca Disease: Generalised Myasthenia Gravis
- The EC has approved Soliris (C5 complement inhibitor) for expanded use to include the treatment of refractory gMG in children & adolescents aged 6-17yrs. who are AChR Ab+. The approval was based on the P-III trial of Soliris in 11 patients aged 12-17yrs. with refractory gMG
- The results showed a clinical benefit in paediatric patients who previously failed immunosuppressive treatment & experienced significant unresolved disease symptoms, improvement in 1EPs of change from baseline in QMG total score were seen at 26wk.
- The efficacy & safety of Soliris was consistent with the established profile of Soliris in clinical trials incl. adults with refractory gMG. Soliris was approved in the US, EU & Japan for adults with NMOSD
Camzyos
Active ingredient: mavacamten Approved: June 27, 2023
Company: BMS Disease: Hypertrophic Cardiomyopathy
- The EC has approved Camzyos (2.5mg, 5mg, 10mg, 15mg capsules) for symptomatic (NYHA, class II-III) obstructive HCM. The approval was based on the 2 P-III trials of Camzyos in 251 & 112 patients
- Both trials met their 1EPs & 2EPs. In (EXPLORER-HCM) trial, 37% achieved composite 1EPs, change from baseline post-exercise LVOT peak gradient; pVO2, improvement of NYHA class ≥1 (65% vs 31%), change from baseline in KCCQ-23 CSS & HCMSQ SoB domain score
- The (VALOR-HCM) trial showed a reduction in composite 1EPs, 82% were no longer eligible for a surgical procedure or not to proceed with SRT after 16wks., 17.9% vs 76.8% proceed with SRT before or at 16wk. or were SRT eligible. In the 2EPs, change from baseline post-exercise LVOT peak gradient, NYHA class improvement of at least 1 class (62.5% vs 21.4%), change from baseline in KCCQ-23 CSS; NT-proBNP & Cardiac Troponin
Opdivo
Active ingredient: nivolumab Approved: June 29, 2023
Company: BMS Disease: Non-Small Cell Lung Cancer
- The EC has approved Opdivo + Pt-based CT for the neoadjuvant treatment of resectable NSCLC at high risk of recurrence in adult patients with tumor cell PD-L1 expression ≥1%
- The approval was based on the P-III trial (CheckMate -816) evaluating Opdivo + CT vs CT alone which showed a clinical improvement in EFS and pCR & the safety profile was consistent with prior reported studies. In all-randomized patients at follow-up of 21mos., m-PFS (31.6 vs 20.8mos.), 24% vs 2.2% achieved pCR, OS showed a 43% reduction in risk of death
- Opdivo + CT was approved for neoadjuvant treatment of patients with resectable NSCLC in 21 countries, incl. the US, Japan & China, and additional regulatory applications are under review by global health authorities
Related Post: Insights+: EMA Marketing Authorization of New Drugs in May 2023
Tags

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.














